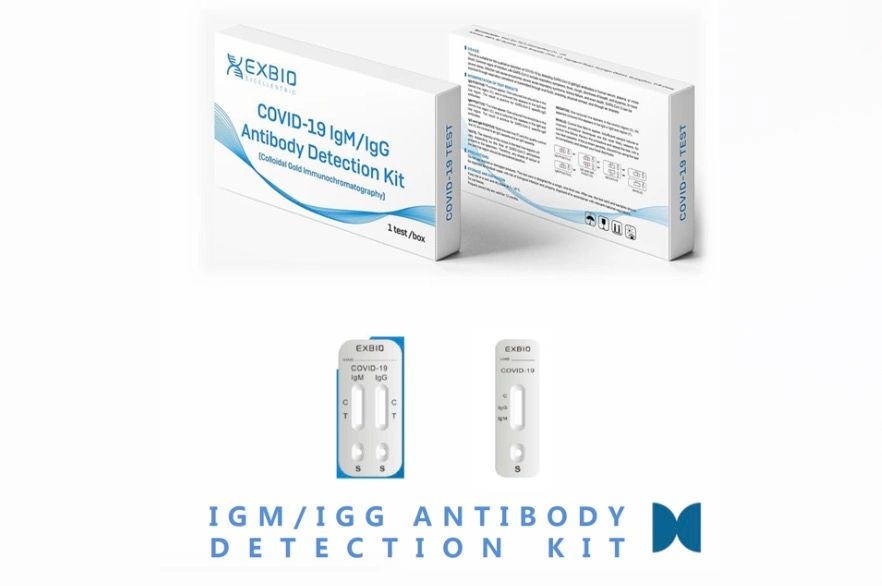
Rapid Test for COVID-19 IgM/IgG Antibody Detection Kit
Qualitative Detection of COVID-19 (SARS-CoV-2) IgM and IgG A
The test uses the principle of colloidal gold immunochromatography and capture method to detect COVID-19 (SARS-CoV-2) IgM and IgG antibodies in human serum, plasma, and whole blood.
PRINCIPLE OF THE TEST

Mechanism
The test uses the principle of colloidal gold immunochromatography and capture method to detect COVID-19 (SARS-CoV-2) IgM and IgG antibodies in human serum, plasma, and whole blood. When the sample contains COVID-19 IgM / IgG antibody and the concentration is greater than or equal to the minimum detection limit, the antibody binds to the antigen immobilized on colloidal gold nanoparticles which migrate to test region 1 (T1) / test region 2 (T2), where it is captured by the secondary antibody to produce a red reaction line. The result is considered positive when a red reaction line appears in either the test region. The result is considered negative when no red reaction line is in the test line region. The test is valid when the control line (C) produces a reaction line and invalid if no control line (C) appears.
Key Features
- Works with venous whole blood, serum or plasma and finger prick whole blood
- A rapid test that takes at most 15 minutes from sample collection to results interpretation
- Precise test results ready in 10 minutes
- Aid in the screening and diagnosis of COVID-19 virus, in combination with PCR
- Room temperature shipping and storage
- No instrument required
- Aid in risk stratification (potential to be used in doctor’s office, ER, airports, schools, etc.): non-immune (IgG/IgM), early infection (IgM only), late infection (IgM/IgG), immune (IgG only)
Simple Procedures
- Collect serum/plasma/blood sample
- Mix serum/plasma sample with diluent and add to sample well (venous) or add blood sample and diluent to sample well (finger prick)
- Read results at 10 minutes

Result Interpretation
- Positive for SARS-CoV-2 IgM:
- A red line appears in test region 1 (T1)/M as well as the control line (C)
- Positive for SARS-CoV-2 IgG:
- A red line appears in the test region 2 (T2)/G as well as the control line (C).
- Positive for Both SARS-CoV-2 IgM and IgG:
- A red line appears in both test region 1 (T1)/M and test region 2 (T2)/G as well as the control line (C).
- Negative for SARS-CoV-2 IgM/IgG:
- A red line appears in the control line (C) but no line appears in test region 1 (T1)/M or test region 2 (T2)/G.
- Invalid Result: the test is invalid if no red line appears in the control line (C).
Limitations of Rapid Test for COVID-19 IgM/IgG Antibody Detection Kit
- This test has not been reviewed by the FDA
- Negative results do not rule out SARS-CoV-2 infection, particularly for those who have been in contact with the virus. Follow up testing by a molecular diagnostic should be considered to rule out infection in these individuals.
- Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status
- Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, OC43, NL63, or 229E.
- Not for the screening of donated blood
FDA NOTIFICATION
Notified the FDA that we have validated and are offering our serology test, COVID-19 (SARS-CoV-2) IgM/IgG Antibody Detection Kit, to commercial laboratory and for POCT settings, in compliance with Section IV.D. of the FDA’s Policy for Diagnostic Tests for Coronavirus Disease-2019. The FDA has not reviewed the validation of this test. Will be pursuing EUA of this test.
Copyright © 2020 C.D.I Corp. U.S. - All Rights Reserved.